Reverse Transcriptase Inhibitors (RTIs) are part of the cocktail of medicines used to treat HIV. So what do they have to do with AGS and why are we starting clinical trials? As of February 2023, there are 3 AGS clinical trials with reverse transcriptase inhibitors either active or about to begin in Europe and the United States. Learn about the theory and why they’re expected to be helpful.
The Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN) FY23 Funding Opportunities
Calling US Health Professionals and Clinical Researchers! The Global Leukodystrophy Initiative (GLIA) seeks to promote collaborative research efforts across the leukodystrophies - now is the time to apply for their Career Development or Pilot Project awards.
GLIA-CTN Pilot Project Award
What is expected: The Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN) wants to grant (2) Pilot Project Awards designed to generate preliminary research data needed to secure more substantial funding through traditional federal, institutional, and/or industry grant mechanisms.
When to apply: Deadline is February 20th, 2023 (check website for details).
Where to apply: https://theglia.org/gliactn/funding-opportunities#fy23pprfa
Why should you apply: The GLIA-CTN Pilot Project Award will provide up to $20,000 for research and salary, inclusive of appropriate fringe and indirect costs allowing you to explore key knowledge gaps with an innovative, early-stage project.
GLIA-CTN Career Development Award
What is expected: The Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN) wants to grant (1) Career Development Award. This is a mentored award designed to provide "protected time" for clinically trained individuals to receive supervised training in biomedical research related to leukodystrophies.
When to apply: Deadline is February 20th, 2023 (check website for details).
Where to apply: https://theglia.org/gliactn/funding-opportunities#fy23cdrfa
Why should you apply: The GLIA-CTN Career Development Award will provide up to $85,000 for research and salary, inclusive of appropriate fringe and indirect costs. The award supports a period of supervised research, in conjunction with career development opportunities, for physician-scientists who require additional mentored training and support during development of an innovative research project.
Questions: Questions regarding the application requirements, submission guidelines, etc. for either award may be directed to GLIA-CTN Program Manager, Omar Sherbini, MPH at email@theglia.org.
Let's find a way forward, together!
AGSAA
Late-Onset AGS
A description of "late-onset" AGS based on 34 children whose disease presented after a year of age.
Late-onset AGS Appears to be More variable
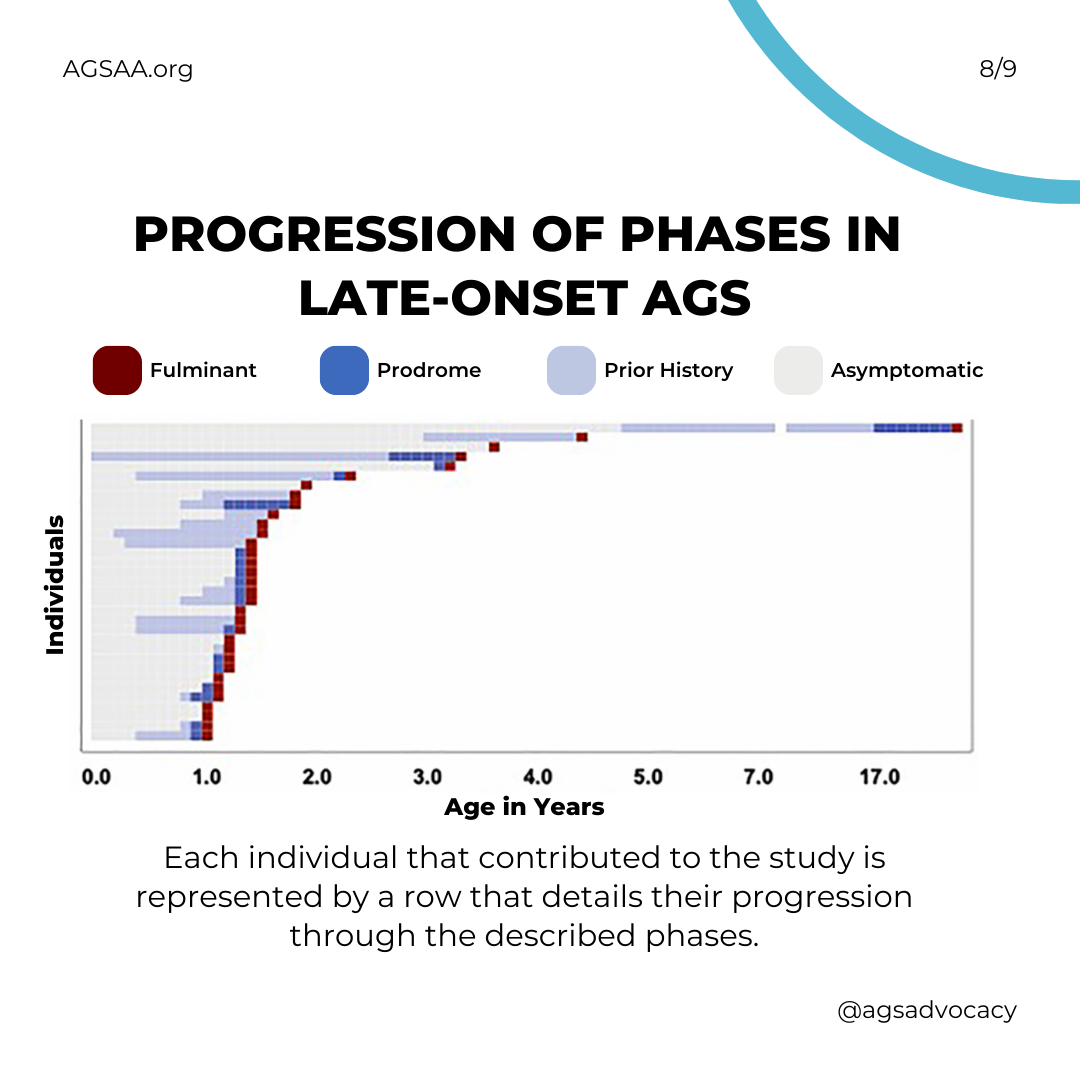
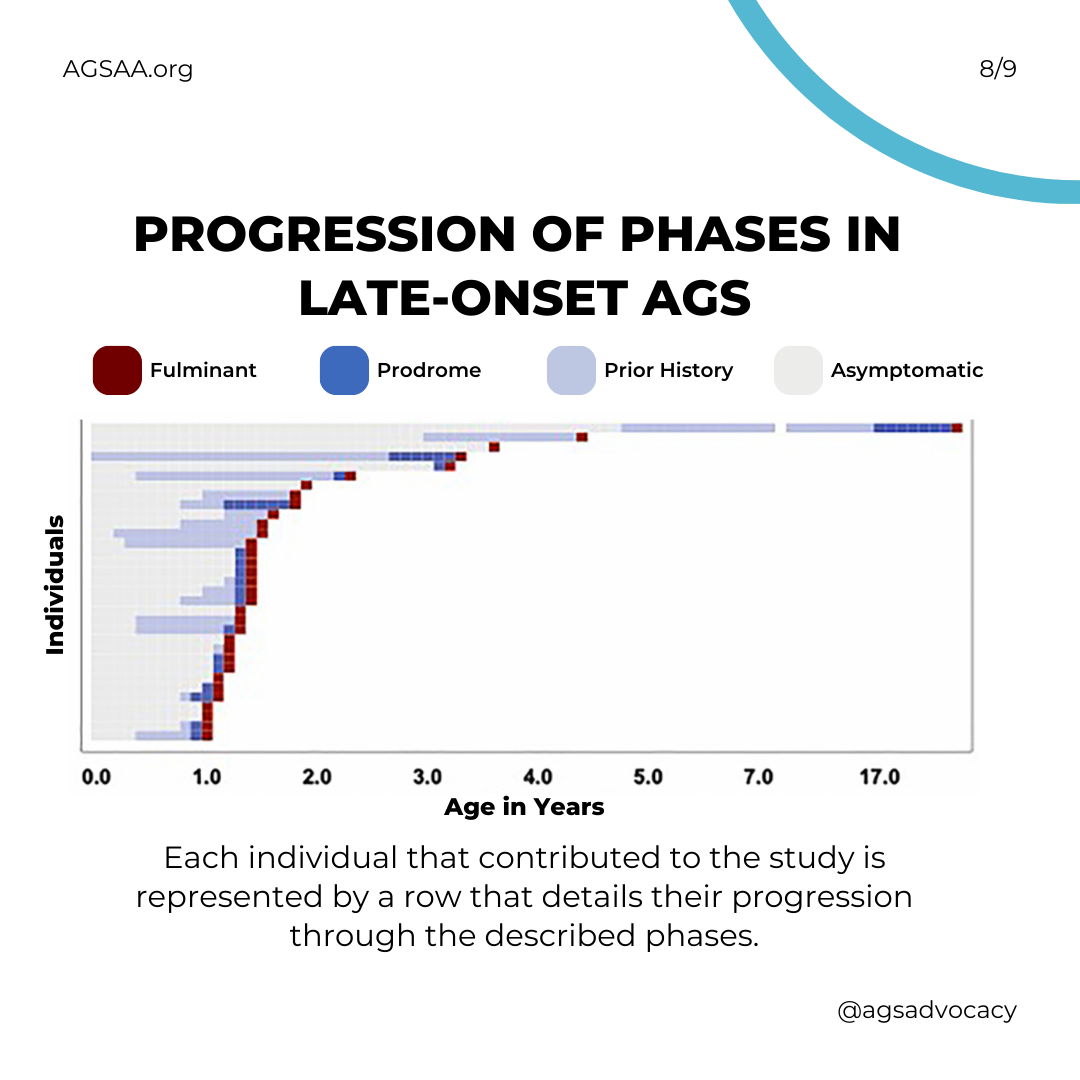
Children with late-onset (aka "atypical") AGS can be different from each other. The clinical course, brain injury, and developmental outcomes of children with late-onset AGS fall on a wider spectrum than that of early infantile onset.
Diagnostic Failures in Atypical AGS Are Common
Diagnosis is complicated by the fact that late-onset children often do not have classical AGS MRI findings (e.g. few have calcifications). While the majority have a medical encounter within a few days of onset, the median time to brain imaging (MRI) is over a month. And, many take over a year to be properly diagnosed. Misdiagnosis is common.
Late-Onset Children Suffer Complications Before Disease Onset
The majority of children with late-onset AGS have a history of AGS-related complications prior to the severe onset of disease. Chilblains, systemic inflammation, irritability, developmental delay and aseptic fevers are common.
Symptoms Worsen Just before AGS HITS
After the early phase, most individuals with atypical AGS demonstrate increasing and worsening features of systemic inflammation during what is called a “prodromal” period. This period typically starts around 1 year and 3 months of age and often includes developmental delay, fevers, irritability, etc.
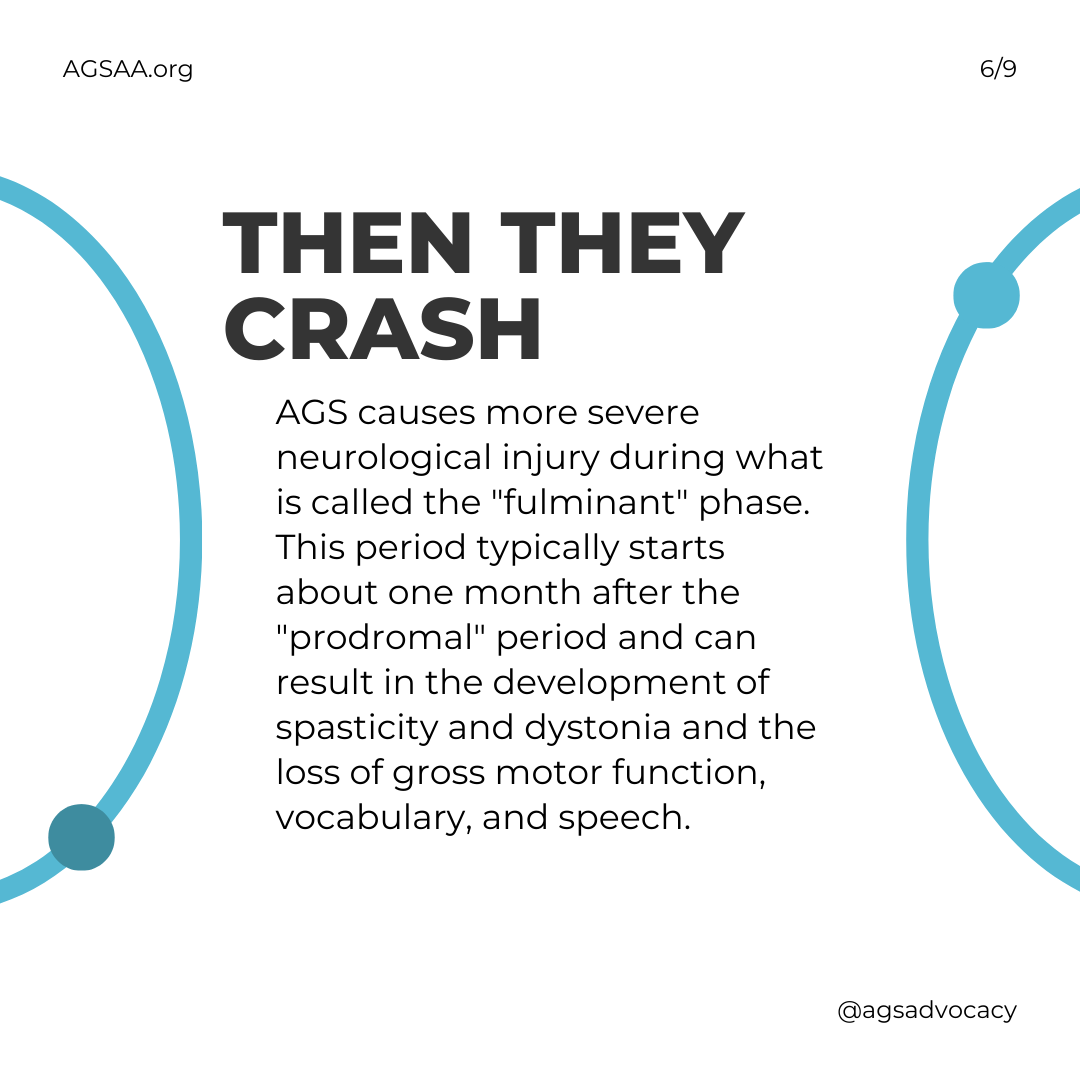
Then They Crash
AGS causes more severe neurological injury during what is called the "fulminant" phase. This period typically starts about one month after the "prodromal" period and can result in the development of spasticity and dystonia and the loss of gross motor function, vocabulary, and speech.
And Wait For Relief
The length of the "fulminant" phase can vary greatly, but about half of children will have experienced the worst of it within 4 weeks. For a few it fade within one week; while for many others (over one-third) it may continue longer and be considered "chronic."